Immune-mediated Diseases
Introduction
Autoimmune diseases such as Systemic Lupus Erythematosus (SLE), Sjögren’s syndrome, and Sarcoidosis steadily increase in prevalence and due to the often chronic disease courses are a major public health concern. In many cases specific treatments are still lacking, have considerable adverse effects, or patients show insufficient response. In order to improve treatment strategies and to potentially develop novel drugs, a better understanding of underlying disease mechanisms is essential.Regulatory T (Treg) cells are a central component of immune regulation and exhibit impaired suppressor functions in various disease settings. Their specific IL-2 receptor (IL2R) configuration consisting of all three IL2R subunits α, β and the common γ chain make them highly sensitive and responsive to low-dose IL-2 therapy compared to conventional CD4+ T (Tcon) cells, which only express the β and γ subunit (Raeber et al., 2023). These characteristics make Treg cells an attractive therapeutic target for various autoimmune diseases.
Insights from the Lab
Our research is focused on understanding underlying pathological mechanisms of autoimmune diseases as well as development and testing of novel therapeutic approaches. In a recent investigator-initiated clinical trial, we studied the effect of low-dose IL-2 treatment in 12 SLE patients. Apart from clinical response, we observed a marked expansion of Treg cells. Multi-omics analysis of these Treg revealed several novel Treg subsets with distinct patterns of activation and migration (Raeber et al., 2022)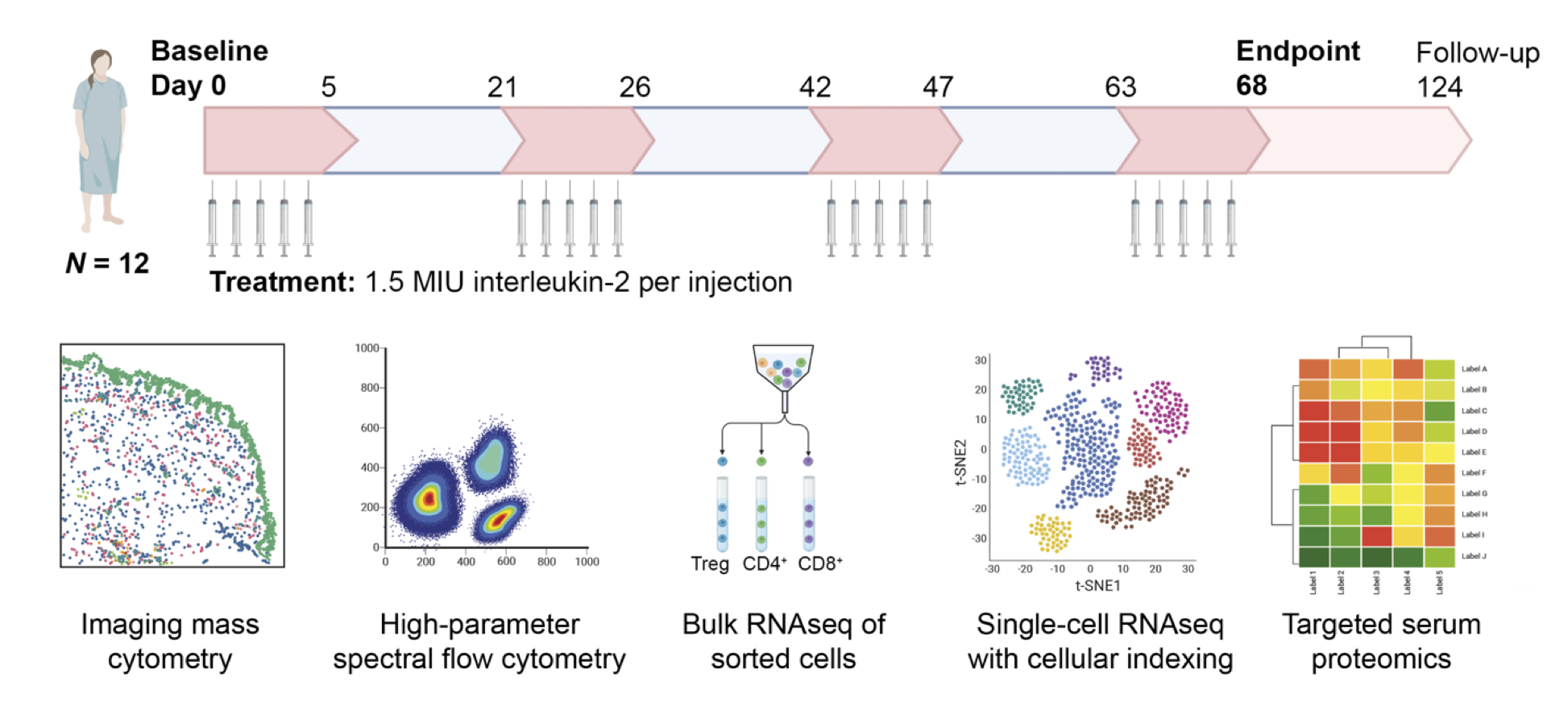
12 patients with Systemic Lupus Erythematosus (SLE) received four cycles (red boxes) of five daily injections of low-dose interleukin-2 (IL-2). Blood samples before and after IL-2 treatment were analyzed with imaging mass cytometry, high-parameter flow cytometry, bulk and single-cell RNA sequencing and targeted serum proteomics.
However, our analysis revealed that not only Treg cells but also effector immune cells including natural killer and conventional CD4+ and CD8+ T cells respond to low-dose IL-2 treatment, limiting treatment efficacy. This motivated us to develop IL-2–anti-IL-2 antibody complexes preferentially shuttling IL-2 to the trimeric IL-2 receptor expressed on Treg cells for clinical application.
Furthermore, we are working on improved diagnostic procedures to reduce invasiveness and increase specificity of diagnosis of immunological disorders. To this end, apart from studying blood components we are additionally analyzing alternative body fluids such as saliva and nasal secretion to gain new insights into the mucosal immunity of patients suffering from Sjögren’s syndrome and other autoimmune diseases.
Furthermore, we are working on improved diagnostic procedures to reduce invasiveness and increase specificity of diagnosis of immunological disorders. To this end, apart from studying blood components we are additionally analyzing alternative body fluids such as saliva and nasal secretion to gain new insights into the mucosal immunity of patients suffering from Sjögren’s syndrome and other autoimmune diseases.
